The dream of being able to predict whether medicine works and its potential side effects—even before you start producing the drug—lives again. This is the potential for new Danish research conducted in collaboration between DTU researchers and the company
Roche Innovation Center Copenhagen.
By Morten Andersen
Over the years, the company has developed a number of drug candidates which have reached late-stage clinical development. The products are targeted at cancer, metabolic disorders and infectious diseases. It may sound incredible that a company with just 65 employees can work with so many different diseases, but this is because the core of its work is a new concept in medical treatment.
The company has now initiated a collaboration with researchers at DTU Physics and DTU Chemistry. The purpose is to take a shortcut in the future development work.
“Today, a large number of almost identical molecules are produced, but most of them are subsequently discarded following laboratory tests. We hope that the research project will eventually enable us to rule out a large number of the substances in advance, because we can better predict their effect and thus focus on those showing the greatest potential,” says Troels Koch, VP Research and CTO at RICC.
Small chemical change, great effect
In this way, you can also reduce the need for laboratory animals. It is an ethical objective, but also to a large extent an economic benefit.
“On the whole, we can initiate our development work at a much higher level. We will probably still produce many different molecules as we do today, but we are far more likely to find the right candidate among them,” says Troels Koch.
He got the idea for the project together with dr.techn. Henrik Bohr, DTU Physics, who from 2001 to 2010 headed the Quantum Protein Centre (Center for Kvanteprotein)—a basic research centre financed by the Danish National Research Foundation. The new project is based on the findings of the centre, and it is carried out in an interdisciplinary collaboration between Bohr's research group and Associate Professor Irene Shim’s research group at DTU Chemistry.
In recent years, more and more research has been conducted in the interface between physics and chemistry. Chemists are among the frequent users of tools from the world of physics, e.g. for studying the atomic structure of a molecule by means of neutron or X-ray scattering. The project carried out in collaboration with RICC is a big step in the direction of industrial application of this academic breakthrough.
Small changes—big consequences
The first scientific presentation of the findings has been published in the journal Nucleic Acid Therapeutics. The article contains six figures that illustrate how changes—that seem quite small from a chemical perspective—result in extensive changes in the structure of the molecules which the company is working with. The figures combine traditional chemical models where the atoms are shown as balls connected by thin bars, which illustrates chemical bonds—with presentations of the electrostatic potential of the molecule surface. The electrostatic potential of the surface can be considered the sum of the positions and potential of the individual electrons. It is of vital importance to the chemical properties of the molecule, and for how the molecules interact with, and in, a biological system.
"The real breakthrough will be that not only will we be able to explain known phenomena, but also to predict the effect and risk of side effects for substances which have not yet been produced,"
dr.techn. Henrik Bohr
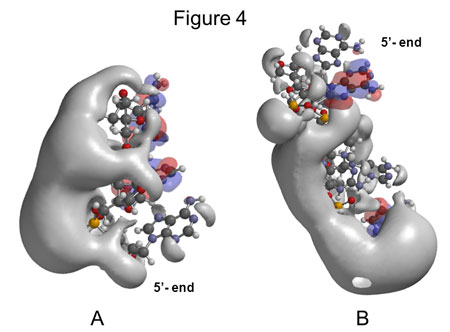
“The figures happen to have a certain similarity with baby elephants so that is what I called them when we first presented the results. Of course, this caused a few laughs, but people were quite aware of the interesting aspect of the findings. Our models can actually explain some of the significant differences observed in the effect of substances which are chemically very closely related,” says Henrik Bohr.
In drug research, there are several examples of substances with chemically identical formulas having different effects—for example in the case of mirror isomers.
"The real breakthrough will be that not only will we be able to explain known phenomena, but also to predict the effect and risk of side effects for substances which have not yet been produced,” adds Henrik Bohr.
Computational heavyweight
The biggest disadvantage of the method is that it is computationally heavy. The ‘baby elephant’ figures are both the result of weeks of computer time at DTU’s supercomputers and laborious work to determine the exact positions of the atoms in large molecules with 2-300 atoms, using electron spectroscopy. In addition to the atoms, we also need to keep track of the electrons, as it is the sum of their potential which creates the local electrostatic potential, as the figures show.
Article from DYNAMO no. 39, DTU's quarterly magazine in Danish.