Mental health is a growing challenge worldwide. This project explores the use of smartphones for continuous monitoring of subjective and objective measures of illness activity in bipolar disorder
Background
Approximately 25% of all people in Europe and the USA experience a mental disorder at least once in their lifetime. Currently monitoring mental disorders relies on subjective clinical self-reporting rating scales, which were developed more than 50 years ago. The EU-funded MONARCA project [1] has established a platform for daily electronic monitoring of subjective and objective measures of illness activity in bipolar disorder using smartphones. The system supports the treatment of bipolar disorder by collecting (i) self-rated (‘subjective’) data on parameters like mood, stress, and cognitive problems, and (ii) automatically sensor-based (‘objective’) data the patients behavior in terms of mobility (GPS), physical activity (accelerometer), social activity (telephony and texting), and general phone usage. This data is captured and forwarded to a server, where it can be accessed, visualized, and monitored by the clinical staff and applied for clinical decision support [2,3,4]. The MONARCA system is now further developed and supported by the company Monsenso.
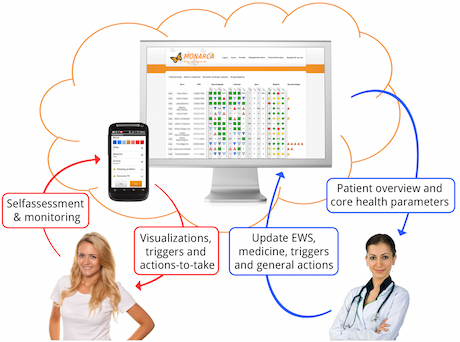
Project Objectives
The MONARCA II project aims to establish clinical evidence on the use of the Monsenso system. The MONARCA II trial [5] uses a randomized controlled single-blind parallel-group design. Patients with bipolar disorder according to ICD-10 who previously have been treated at the Copenhagen Clinic for Affective Disorder, Denmark are included and randomized to either daily use of the Monsenso system including an feedback loop between patients and clinicians (the intervention group) or to the use of a smartphone for normal communicative purposes (the control group) for a 9-month trial period. The trial was started in September 2014 and recruitment is ongoing.
The outcome measures between the intervention group and the control group are:
- differences in depressive and manic symptoms; rate of depressive and manic episodes (primary);
- automatically generated objective data on measures of illness activity; number of days hospitalized; psychosocial functioning (secondary);
- perceived stress; quality of life; self-rated depressive symptoms; self-rated manic symptoms; recovery; empowerment and adherence to medication (tertiary)
Ethical permission has been obtained. Positive, neutral and negative findings will be published.
If the system is effective in reducing depressive and/or manic symptoms (and other symptoms of bipolar disorder) and the rate of episodes, there will be basis for extending the use to the treatment of bipolar disorder in general and in larger scale.
References
- F Gravenhorst et al. Mobile phones as medical devices in mental disorder treatment: an overview. Personal and Ubiquitous Computing, 19(2):335-353, 2015. [pdf]
- JE Bardram et al. The MONARCA self-assessment system: a persuasive personal monitoring system for bipolar patients. In Proceedings of the 2nd ACM SIGHIT International Health Informatics Symposium. p 21-30, NY, USA, 2012. [pdf]
- JE Bardram et al. Designing mobile health technology for bipolar disorder: a field trial of the MONARCA system. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems. p. 2627-2636, NY, USA, 2013. [pdf]
- M Frost et al. Supporting disease insight through data analysis: refinements of the MONARCA self-assessment system. In Proceedings of the 2013 ACM international joint conference on Pervasive and ubiquitous computing. p. 133-142, USA, 2013. [pdf]
- M Faurholt-Jepsen et al. Daily electronic monitoring of subjective and objective measures of illness activity in bipolar disorder using smartphones - the MONARCA II trial protocol: a randomized controlled single-blind parallel-group trial. BMC psychiatry, 14(1):309, 2014. [pdf]